| Name | Hwang-Verslues, Wendy W. Ph.D. |
|---|---|
| Incumbent | Associate Professor |
| 所屬單位 |
Ovarian cancer (OC), high-grade serous ovarian carcinoma (HGSOC), ovarian clear cell carcinoma (OCCC), tumor microenvironment (TME), cancer metabolism, organotropic metastasis, circadian clock, BMAL1, BMAL2, PER2, chronotherapy. Ph.D., Environmental Toxicology, University of California-Riverside, CA, U.S.A., 2002-2007 Master of Environmental Management, Duke University, Durham, NC, U.S.A., 2000-2002 Bachelor of Science, Department of Geography, National Taiwan University, Taipei, Taiwan, 1995-1999 |
| NCLscan: |
|
| 研究方向/領域 |
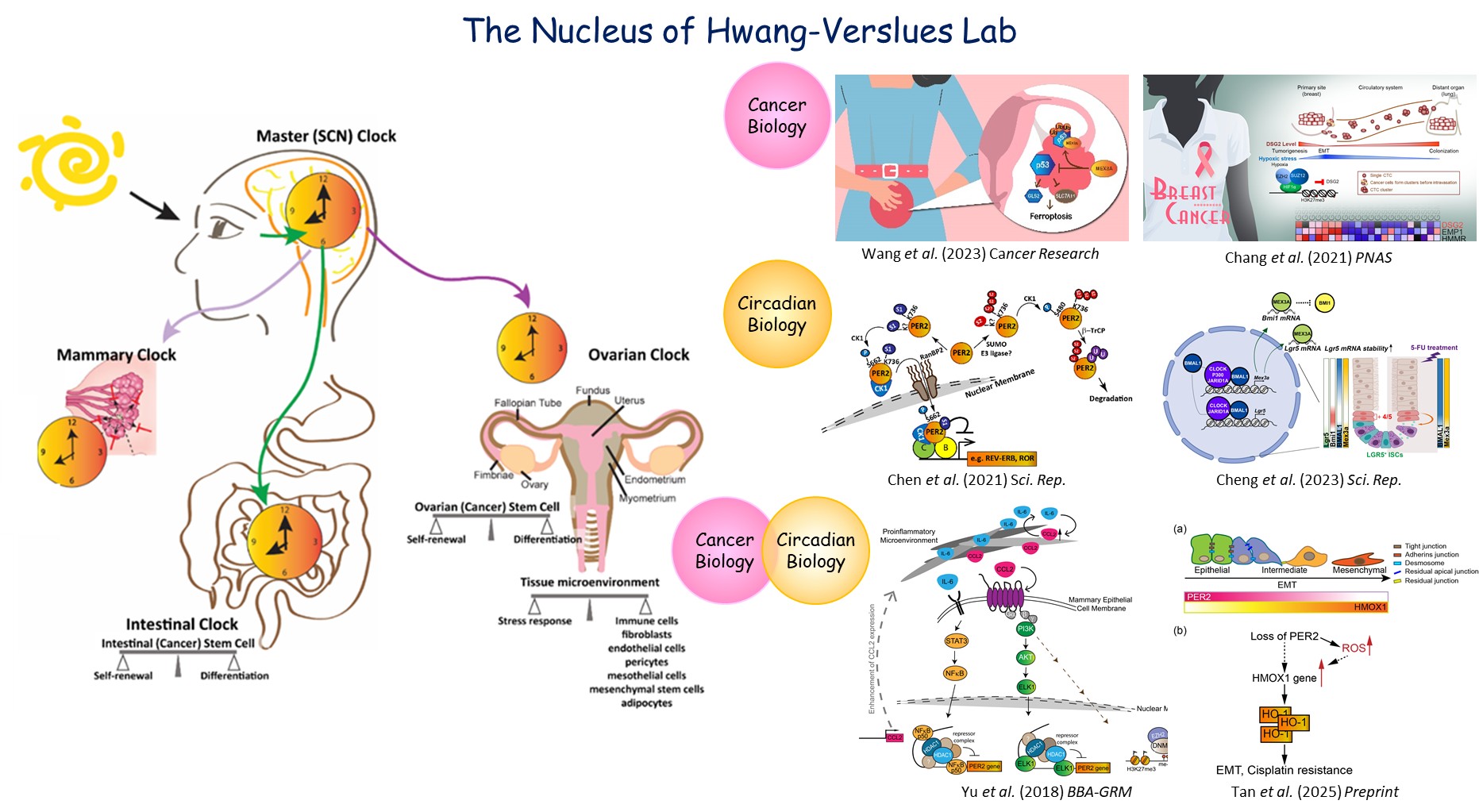
RESEARCH INTERESTSMy research has centered on identification of novel cancer cell-intrinsic and -extrinsic factors critical for tumor initiation, progression, metastasis and recurrence. The long-term vision is to utilize this fundamental mechanistic research to facilitate the development of precision cancer medicine. I specifically focus on ovarian cancer (OC) and breast cancer (BC). My team has demonstrated that MEX3A, an RNA-binding/E3 ligase dual-function protein, is crucial for OC tumorigenesis. MEX3A-mediated p53 protein degradation prevented ferroptosis and enhanced tumorigenesis of OC expressing wild type (wt) p53 [Wang et al, Cancer Research, 2023]. We also found a cell adhesion molecule, Desmoglein2 (DSG2), whose dynamic repression and de-repression in response to changing levels of oxygen allowed BC cells to detach from hypoxic primary tumor, invade in circulation and later colonize in oxygen rich distal organs [Chang et al, PNAS, 2021]. I have also made major contributions in understanding the functions of circadian core clock genes, such as PERIOD2 (PER2), in BC initiation and progression [Yu et al, BBA-GRM, 2018; Hwang-Verslues et al, PNAS, 2013]. Recently, my laboratory found that PER2 controls ROS homeostasis and HO-1 (Heme oxygenase-1, HMOX1) expression in OC cells. Loss of PER2 drove epithelial-like OC cells to undergo EMT and increased cisplatin resistance [Preprint DOI: 10.21203/rs.3.rs-5242949/v1].
Ongoing Research — OC heterogeneity, metabolism, organotropic metastasis and drug resistance. OC is the deadliest gynecological cancer due to its high heterogeneity, metastasis, chemoresistance and recurrence. Unfortunately, the underlying mechanisms crucial for OC malignancy remain elusive. High grade serous ovarian carcinoma (HGSOC) is the most prevalent subtype and has been the most studied. Ovarian clear cell carcinoma (OCCC) is the second most prevalent subtype. OCCC incidence rate is particularly high, and rising, in Asian populations, but it remains less understood. HGSOC is more aggressive than other subtypes, has greater genomic instability, expresses mutated (mut) p53 and BRCA1/2, and easily develops resistance to the standard OC chemotherapy. In contrast, OCCC has wt p53 and low BRCA mutation frequency. While OCCC may not be as aggressive in early stage, it generally has higher metastasis and worse prognosis in advanced stages compared to HGSOC. Numerous treatment strategies have been evaluated for OCCC, but the overall efficacy and subtype selectivity are low. Developing combinations of targeted therapy and immunotherapy tailored to specific OC subtypes is promising but requires understanding of cancer cell intrinsic mechanisms and tumor microenvironment (TME) interactions that is still lacking for HGSOC and OCCC. The goal of our ongoing research is to develop such understanding of HGSOC and OCCC tumorigenesis, metastasis and TME interactions, with focus on the role of MEX3A and BMAL2, a circadian gene that we recently found to have a prominent role in both HGSOC and OCCC.
|
EDUCATION AND POSITIONS HELD
- 2023-Present, Associate Professor, Genomics Research Center, Academia Sinica, Taipei, Taiwan
- 2015-2023, Assistant Professor, Genomics Research Center, Academia Sinica, Taipei, Taiwan
- 2022-Present, Committee Member, Taiwan FDA Genetically Modified Foods Review Group, Taiwan
- 2022-Present, Committee Member, Academia Sinica Institutional Animal Care and Use Committee, Taiwan
- 2022-Present, Member, Academia Sinica Animal Experimentation Panel of Experts, Taiwan
- 2020-Present, Committee Member, Academia Sinica Institutional Review Board on Biomedical Science Research, Taiwan
- 2020-Present, Program Coordinator of Genomics Research Center (Academia Sinica)-China Medical University Cancer Biology Graduate Program, Taiwan
- 2017-Present, Adjunct Assistant Professor, Ph.D. Program for Cancer Molecular Biology and Drug Discovery, Taipei Medical University, Taiwan
- 2017-Present, Grant reviewer for National Science and Technology Council (NSTC), Taiwan
- 2017-2019, Keystone Symposia Programming Consultant Study Group member, USA
- 2017-Present, Member, Society for Research on Biological Rhythms, USA
- 2006-2007, Instructor, Department of Public Health, Loma Linda University, Loma Linda, CA, USA
- 2005-Present, Member, American Association for Cancer Research, USA
HONORS
- 2013, Postdoctoral Fellow Academic Publication Award, Taiwan National Science Council, Taiwan
- 2013, The 72nd Annual Meeting of the Japanese Cancer Association Travel Grant Award, Japan
- 2012, The 1st GRC Best Performance-and-Service Award, Genomics Research Center, Academia Sinica, Taiwan
- 2011, The 70th Annual Meeting of the Japanese Cancer Association Travel Grant Award, Japan
- 2010-2011, Academia Sinica Postdoctoral Research Fellowship, Taiwan
- 2008-2009, Academia Sinica Distinguished Postdoctoral Scholar Fellowship, Taiwan
- 2007, Sigma Xi Grants-in-Aid of Research Award, USA
- 2006, Fukuto Award, University of California-Riverside, USA
- Cheng L.-T., Tan G.Y.T., Chang F.-P., Wang C.-K., Chou Y.-C., Hsu P.-H., Hwang-Verslues W.W.*, 2023, “Core clock gene BMAL1 and RNA-binding protein MEX3A collaboratively regulate Lgr5 expression in intestinal crypt cells”, SCIENTIFIC REPORTS, 13, 17597. (SCIE)
- Wang C.-K., Chen T.-J., Tan G.Y.T., Chang F.-P., Sridharan S., Yu C.-H. A., Chang Y.-H., Chen Y.-J., Cheng L.-T., Hwang-Verslues W.W.*, 2023, “MEX3A mediates p53 degradation to suppress ferroptosis and facilitate ovarian cancer tumorigenesis”, Cancer Research, 83 (2), 251-263. (SCIE)
- Chen L.C., Hsieh Y.L., Tan G.Y.T., Kuo T.Y., Chou Y.C., Hsu P.H., Hwang-Verslues W.W.*, 2021, “Differential Effects of SUMO1 and SUMO2 on Circadian Protein PER2 Stability and Function”, SCIENTIFIC REPORTS, 11, 14431. (SCIE)
- Chang P.H., Chen M.C., Tsai Y.P., Tan G.Y.T., Hsu P.H., Jeng Y.M., Tsai Y.F., Yang M.H., Hwang-Verslues W.W.*, 2021, “Interplay between Desmoglein2 and hypoxia controls metastasis in breast cancer”, PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES OF THE UNITED STATES OF AMERICA, 118(3), e2014408118. (SCIE)
- Fararjeh, A., Tu, S.H., Chen, L.C., Cheng, T.C., Liu, Y.R., Chang, H.L., Chang, H.W., Huang, C.C., Wang, H.C.R., Hwang-Verslues, W.W., Wu, C.H., Ho, Y.S.*, 2019, “Long-term exposure to extremely low-dose of nicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) induce non-malignant breast epithelial cell transformation through activation of the a9-nicotinic acetylcholine receptor-mediated signaling pathway”, ENVIRONMENTAL TOXICOLOGY, 34(1), 73-82. (SCIE)
- Yu, C.-W., Cheng, K.-C., Chen, L.-C., Lin, M.-X., Chang, Y.-C., Hwang-Verslues, W.W.*, 2018, “Pro-inflammatory cytokines IL-6 and CCL2 suppress expression of circadian gene Period2 in mammary epithelial cells”, BIOCHIMICA ET BIOPHYSICA ACTA-GENE REGULATORY MECHANISMS, 1861(11), 1007-1017. (SCIE)
- Abdul F.F.-S., Tu S.-H., Chen L.-C., Liu Y.-R., Lin Y.-K., Chang H.-L., Chang H.-W., Wu C.-H., Hwang-Verslues W.W., Ho Y.-S., 2018, “The Impact of the effectiveness of GATA3 as a prognostic factor in breast cancer”, Human Pathology, 80, 219-230. (SCIE)
- Wu YW, Hsu KC, Lee HY, Huang TC, Lin TE, Chen YL, Sung TY, Liou JP, Hwang-Verslues WW, Pan SL, Huang-Fu WC,, 2018, “A novel dual HDAC6 and tubulin inhibitor, MPT0B451, displays anti-tumor ability in human cancer cells in vitro and in vivo”, FRONTIERS IN PHARMACOLOGY, 9, 205. (SCIE)
- Huang S.-C., Wei P.-C., Hwang-Verslues W.W., Kuo W.-H., Jeng Y.-M., Hu C.-H., Shew J.-Y., Huang C.-S., Chang K.-J., Lee E. Y.-H. P., Lee W.-H.*, 2017, “TGF-β1 secreted by Tregs in lymph nodes promotes breast cancer malignancy via up-regulation of IL-17RB”, EMBO MOLECULAR MEDICINE, 9(12), 1660-1680. (SCIE)
- Cheung, S.K.C., Chuang, P.-K., Huang, H.-W., Hwang-Verslues, W.W., Cho C.H.-H., Yang, W.-B., Shen, C.-N., Hsiao, M., Hsu, T.-L., Chang, C.-F., Wong, C.-H., 2016, “Stage-Specific Embryonic Antigen-3 (SSEA-3) and β3GalT5 are Cancer Specific and Significant Markers for Breast Cancer Stem Cells”, Proceedings of the National Academy of Sciences of the United States of America, 113(4), 960-965. (SCIE)
- Wu, H.-H*, Hwang-Verslues, W.W.*, Lee, W.-H., Huang, C.-K., Wei, P.-C., Chen, C.-L. , Shew, J.-Y., Lee, E. Y.-H. P., Jeng, Y.-M., Tien, Y.-W., Ma, C., Lee, W.-H., 2015, “Targeting IL-17B/RB signaling with an anti-IL17RB antibody blocks pancreatic cancer metastasis by silencing multiple chemokines”, JOURNAL OF EXPERIMENTAL MEDICINE, 212(3), 333-349. (SCIE)
- Hwang-Verslues WW, Chang PH, Jeng YM, Kuo WH, Chiang PH, Chang YC, Hsieh TH, Su FY, Lin LC, Abbondante S, Yang CY, Hsu HM, Yu JC, Chang KJ, Shew JY, Lee EY, Lee WH, 2013, “Loss of corepressor PER2 under hypoxia up-regulates OCT1-mediated EMT gene expression and enhances tumor malignancy.”, Proceedings of the National Academy of Sciences of the United States of America, 110(30), 12331-6. (SCIE)
- Chang, P.-H., Hwang-Verslues, W.W., Chang, Y.-C., Chen, C.-C., Hsiao, M., Jeng, Y.-M., Chang, K.-J., Lee, E. Y.-H. P., Shew, J.-Y., Lee, W.-H., 2012, “Activation of Robo1 signaling of breast cancer cells by Slit2 from stromal fibroblast restrains tumorigenesis via blocking PI3K/Akt/β-catenin pathway”, CANCER RESEARCH, 72(18), 4652-4661. (SCIE)
- Hwang-Verslues, W.W.*, Lee, W.-H., Lee, E.Y.-H.P., 2012, “Biomarkers to target heterogeneous breast cancer stem cells (invited review)”, Journal of Molecular Biomarkers & Diagnosis, S8, 006.
- Hwang-Verslues, W.W., Chang P.-H., Wei P.-C., Yang C.-Y., Huang C.-K., Kuo W.-H., Shew J.-Y., Chang K.-J., Lee E.-Y., Lee W.-H., 2011, “miR-495 is upregulated by E12/E47 in breast cancer stem cells, and promotes oncogenesis and hypoxia resistance via downregulation of E-cadherin and REDD1”, ONCOGENE, 30(21), 2463-2474. (SCIE)
- Hwang-Verslues, W.W., Sladek, F.M., 2010, “HNF4a-role in drug metabolism and potential drug target? (review)”, CURRENT OPINION IN PHARMACOLOGY, 10(6), 698-705. (SCIE)
- Bolotin E., Liao H., Ta T.C., Yang C., Hwang-Verslues W.W., Evans J.R., Jiang T., Sladek F.M., 2010, “Integrated approach for identification of HNF4α target genes using protein binding microarrays”, HEPATOLOGY, 51(2), 642-653. (SCIE)
- Hwang-Verslues, W.W., Kuo W.-H., Chang P.-H. Pan C.-C., Wang H.-H., Tsai S.-T., Jeng Y.-M., Shew J.-Y., Kung J.T, Chen C.-H., Lee E. Y.-H. P., Chang K.-J., Lee W.-H., 2009, “Multiple lineages of human breast cancer stem/progenitor cells identified by profiling with stem cell markers”, PLoS One, 4(12), e8377. (SCIE)
- Hwang-Verslues, W.W., Chang, K.J., Lee, E.Y-H.P., Lee, W.-H., 2008, “Breast cancer stem cells and tumor suppressor genes”, Journal of Formosan Medical Association, 107(10), 751-766.
- Hwang-Verslues, W.W., Sladek, F.M., 2008, “Nuclear receptor HNF4alpha1 competes with oncoprotein c-Myc for control of the p21/WAF1 promoter”, MOLECULAR ENDOCRINOLOGY, 22(1), 78-90.
- Maeda, Y.*, Hwang-Verslues, W.W.*, Wei, G., Fukazawa, T., Durbin, M.L., Owen, L. B., Liu, X. and Sladek, F.M., 2006, “Tumor suppressor p53 down regulates the expression of the human hepatocyte nuclear factor 4alpha (HNF4a) gene”, BIOCHEMICAL JOURNAL, 400(2), 303-313. (SCIE)
Breast cancer:
https://www.scitw.cc/posts/hotnews16299
https://health.ltn.com.tw/article/breakingnews/3432015
Ovarian cancer:
https://www.sinica.edu.tw/tw/News_Content/36/528
Circadian and Cancer:
https://www.natgeomedia.com/science/article/content-9412.html
https://srbr.org/a-link-between-clocks-and-cancer/
https://investigator.tw/13082/